Find out what the user can do to reduce battery corrosion and shedding
Corrosion occurs primarily on the grid, and it is known as a “softening and shedding” of the lead off the plates. This reaction cannot be avoided because the electrodes in a lead acid environment are always reactive. Lead shedding is a natural phenomenon that can be reduced but not eliminated. A battery that reaches the end of life through this failure mode has met or exceeded the anticipated life span. Limiting the depth of discharge, reducing the cycle count, operating at a moderate temperature and controlling overcharge are preventive measures to keep corrosion in check.
To reduce corrosion on long-life batteries, manufacturers keep the specific gravity at a moderate 1.200 level when fully charged, compared to 1.265 and greater for high-performance lead acid batteries(See BU-903: How to measure State-of-charge) A lower specific gravity decreases the specific battery energy.
Applying prolonged overcharge is another contributor to grid corrosion. This is especially damaging to sealed lead acid systems. While the flooded lead acid has some resiliency to overcharge, sealed units must operate at the recommended float charge(See BU-403: Charging Lead Acid)
Chargers with variable float voltages adjust the charge voltage to the prevailing temperature. Reducing the float charge when the ambient temperature reaches 29°C (85°F) and increasing it when colder lowers corrosion(See BU-410: Charging at High and Low Temperatures) Most chargers for stationary batteries feature temperature control, but this not common in vehicles. A fully charged starter battery is kept at 14.40V (2.40V/cell) while driving and this can lead to overcharge. The recommended float voltage is 13.60V (2.27V/cell).
As lead acid batteries are being replaced with Li-phosphate (LiFePO), precise charging is paramount. While the automotive charging system provides the correct end-of-charge voltage for LiFePO, Li-ion should receive no further charge when the battery is fully charged. With the LiFePO replacement, this does not happen and the starter battery receives continuous charge while cruising. Although LiFePO is more tolerant to overcharge than cobalt-blended Li-ion, overcharge can shorten the life of the Li-phosphate battery.
To attain maximum surface area, the lead on a starter battery is applied in a sponge-like form. With time and use, chunks of lead fall off and reduce the performance. Figure 1 illustrates the innards of a corroded lead acid battery.
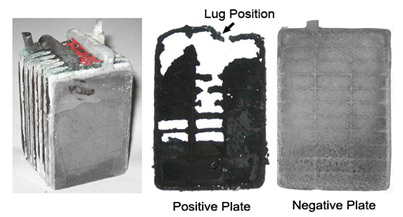
Grid corrosion is unavoidable because the electrodes in a lead acid environment are always reactive. Lead shedding is a natural phenomenon that can only be slowed and not eliminated.
The terminals of a battery can also corrode. This is often visible with the formation of white powder as a result of oxidation between two different metals connecting the poles. Terminal corrosion can eventually lead to an open electrical connection. Changing the connecting terminals to lead, the same material as the battery pole of a starter battery, will solve most corrosion problems.
The lead within a battery is mechanically active. On discharge, the lead sulfate causes the plates to expand, a movement that reverses during charge when the plates contract again. Over time, sulfite crystals form that cause shedding of lead material. The shedding in a starter battery is manageable because the battery does not go through a deep discharge, but this is a larger problem with a deep-cycle battery.
Electrical short is another failure mode, especially with starter batteries in trucks. As the battery sheds its lead to the bottom of the container, a conductive layer forms that gradually fills the allotted space in the sediment trap. In time, the now conductive liquid may reach the plates, creating a shorting effect. The term “short” is a misnomer and elevated self-discharge or soft short would be better terms to describe this condition.
Soft shorts are difficult to detect because the battery functions normally immediately after charge and everything seems fine. In essence, a charge wipes out all evidence of a soft short condition, except perhaps an elevated temperature during charge that may be noticed when touching the battery housing. However, once rested for 6–12 hours, the battery begins to show anomalies such as a lower open circuit voltage and reduced specific gravity.
The measured capacity will also be low because self-discharge has consumed some of the stored energy. According to the 2010 BCI Failure Mode Study, shorted batteries accounted for 18 percent of battery failures, a drop from 31 percent 5 years earlier. Improved manufacturing methods may account for this reduction.
Another form of soft short is mossing. This occurs when the separators and plates are slightly misaligned as a result of poor manufacturing practices and they cause parts of the plates to become naked. Such exposure promotes the formation of conductive crystal moss around the edges, which leads to elevated self-discharge.
Lead drop is another cause of short in which chunks of lead break loose from the welded bars connecting the plates. Unlike a soft short that develops with wear and tear, a lead drop often occurs early in battery life due to a manufacturing defect. This can lead to a serious electrical short with a permanent voltage drop that could result in thermal runaway.
The most radical and serious form of short is a mechanical failure in which the suspended plates become loose and touch each other. This results in a sudden high discharge current that can lead to excessive heat buildup and thermal runaway. Sloppy manufacturing as well as excessive shock and vibration are the most common contributors to this failure.
Reference
[1] Source: Journal of Power Sources (2009)

Looking for comments from the previous website?
Comments from the previous website are not compatible with our new commenting system but we have preserved them so our users can still reference and make use the information in them.

What is the main reason for heavy Oxidation in Battery the lug below the strap.