Capacity is the leading health indicator of a battery, but estimating it on the fly is complex. The traditional charge/discharge/charge cycle is still the most dependable method to measure battery capacity. While portable batteries can be cycled relatively quickly, a full cycle on large lead acid batteries is not practical for capacity measurement.
SAE (Society of Automotive Engineers) specifies the capacity of a starter battery by Reserve Capacity (RC). RC reflects the runtime in minutes at a steady discharge of 25A. DIN (Deutsches Institut für Normung) and IEC (International Electrochemical Commission) mark the battery in Ah at a typical discharge of 0.2C-rate (5h ate) for starter batteries. A 60Ah battery would discharge at 12A. No accurate RC to Ah conversion exists but the most common formula is RC divided by 2 plus 16. A short method is dividing RC by 1.9.
Discharge Method
One would assume that capacity measurement by discharge is the most accurate method, but this is not always the case, especially with lead acid batteries. Even when using highly accurate equipment in a temperature controlled environment and following established charge and discharge standards, variations between identical tests occur. This is not fully understood except to realize that batteries are electrochemical devices that have human-like qualities. Our IQ level also varies depending on the time of day and other conditions. Lithium- and nickel-based chemistries provide more consistent discharge results than lead acid.
Cadex labs checked 91 starter batteries with diverse performance levels, and the results have been plotted in Figure 1. The horizontal x-axis presents the batteries from weak to strong, and the vertical y-axis reflects the capacity. The tests followed SAE J537 standards by applying a full charge and a 24-hour rest, followed by a regulated 25A discharge to 10.50V (1.75V/cell). The results in diamonds represent Test 1. The test was repeated under identical conditions, and the capacities shown in squares characterize Test 2. Only done within days of each other, Test 1 and 2 differ much as +/-15 percent average in capacity. Other laboratories observe similar discrepancies.
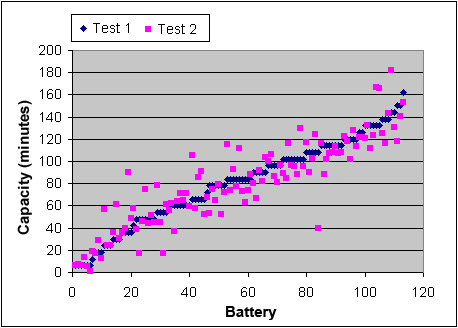
The capacities differ +/–15% between Test 1 and Test 2. Tests were done according to SAE J537
When evaluating battery test results, the question is asked: “Against what standard are the readings compared?” If done with the classic charge/discharge cycle that has large inaccuracies, then modern test technologies have no benchmark and scientists may ask: “Which method is more accurate, the discharge/charge method or other evolving technologies?” This is a valid question as non-intrusive technologies are emerging that only take seconds to test a battery.
Non-invasive Method
Spectro™ (by Cadex) uses multi-model electrochemical impedance spectroscopy (EIS) that checks battery health in seconds with a scanning process. The non-invasive technology combines EIS with complex modeling to estimate capacity, CCA and SoC with the help of matrices, also known as look-up tables. Here is how it works:
A sinusoidal signal of multiple frequencies is injected into the battery at a few millivolts. After digital filtering, the extracted signal forms a Nyquist plot onto which various electro-chemical models are superimposed. Spectro™ selects the best matching models; non-fitting replicas are rejected. Data fusion then correlates the values of the key parameters to derive at capacity and CCA estimations. Figure 2 illustrates the patented process in a simplified way.
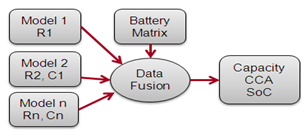
A sinusoidal signal produces a Nyquist plot; data fusion correlates the values of the key parameters to estimate capacity and CCA.
The Nyquist plot was invented by Harry Nyquist (1889–1976) while at Bell Laboratories. It presents the frequency response of a linear system displaying both amplitude and phase angle on a single plot using frequency as parameter. The horizontal x-axis of a Nyquist plot reveals the real ohm impedance while the vertical y-axis represents the imaginary impedance(See BU-907: Testing Lithium-based Batteries)
Capacity vs. CCA
Starter batteries have two distinct values, CCA and capacity. These two readings are different; one cannot predict the other and correlation between the two is almost non-existent, except perhaps towards the end of battery life(See BU-806, Tracking Battery Capacity and Resistance as part of Aging)
Most rapid-testers look at the internal resistance and do a CCA approximation. Reading battery resistance is relatively simple, but this alone cannot predict capacity, nor can it tell when to replace a battery as the end-of-life characteristic is primarily capacity related. Most starter batteries crank the engine with very little capacity; a sudden failure might occur when the capacity drops below 30 percent.
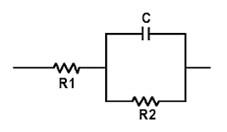
Some battery testers, including Spectro™, display “Resistance High” when the ohmic reading is elevated, a failure that commonly relates to heat damage. A working starter battery reflects a single-digit mOhm value that is represented by R1 in the Randles model on the right(See BU-902: How to Measure Internal Resistance) Batteries developing high resistance move into double-digit readings, and these can be caused by these conditions:
- Low electrolyte level(See BU-804c: Water Loss, Acid Stratification and Surface Charge)
- Stratification of electrolyte(See BU-804c: Water Loss, Acid Stratification and Surface Charge)
- Sulfation of electrodes(See BU-804b: Sulfation and How to Prevent it)
- Bad or deteriorated weld connections of the collector plates and posts
- Collector plate cracking corroded(See BU-804a: Corrosion, Shedding and internal Short)
- Poor battery connection at the clamps or internal to the battery
R1 represents the electrolyte resistance, which is affected by items 1 and 2 above. Items 3 to 6 relate R1 characterizes the electrolyte resistance created by low electrolyte and/or acid stratification as reflected in items 1 and 2 of the above listed conditions. Items 3 to 6 relate to sulfation, corrosion and contact resistance from the battery posts to the electrodes as well as the electrodes to the electrolyte.
The R2/C parallel circuit represents charge transfer resistance and speed. It signifies the required energy needed to overcome the potential barrier at the electrode-electrolyte interface that activates the ion inside the electrolyte, which results in moving electrons from the electrode to the terminals. On a poor battery, the barrier resistance is higher than in a good battery with high capacity. The R2/C branch holds the secret to capacity estimation and differ from the more mechanical conditions captured in R1.
The ability to separate individual components in the Randles model, as Spectro™ does, enables improved battery assessment that reduces battery replacement, especially during the warranty period. “Resistance High” distinguishes a battery with low charge from one that has a genuine defect. The test can be done with a partial charge.
“How accurate are the readings?” car mechanics ask. This depends on the battery. A fault can only be diagnosed with confidence if clear symptoms are present. A new battery or one that has been in storage can become an outlier on capacity estimation. Best results are achieved with a “working” battery that is pulled from service. Accuracy is also based on the quality of the matrix(See BU-905: Testing Lead Acid Batteries, Matrix).
Although capacity and CCA readings are clearly marked on the battery, these values are not always correct. The CCA of some starter batteries are found to be higher or lower than shown; only the manufacturer would know. Because of high cost, CCA tests are seldom done once the battery is sold. In addition, deep-cycle batteries show low capacity readings when new and this could lead to warranty returns. The values will increase as the battery is being formatted with use(See BU-701: How to Prime Batteries)
References
[1] Courtesy of Cadex (2005)
[2] J. Tinnemeyer, "Fuzzy logic method and apparatus for battery state of health determination". US Patent US7072871B1, 4 07 2006-07-04.

Looking for comments from the previous website?
Comments from the previous website are not compatible with our new commenting system but we have preserved them so our users can still reference and make use the information in them.

Good day how do I refurbish a e-bike battery 12v/20amp 3 years old not pregnant clean looking