Invented by the French physician Gaston Planté in 1859, lead acid was the first rechargeable battery for commercial use. Despite its advanced age, the lead chemistry continues to be in wide use today. There are good reasons for its popularity; lead acid is dependable and inexpensive on a cost-per-watt base. There are few other batteries that deliver bulk power as cheaply as lead acid, and this makes the battery cost-effective for automobiles, golf cars, forklifts, marine and uninterruptible power supplies (UPS).
The grid structure of the lead acid battery is made from a lead alloy. Pure lead is too soft and would not support itself, so small quantities of other metals are added to get the mechanical strength and improve electrical properties. The most common additives are antimony, calcium, tin and selenium. These batteries are often known as “lead-antimony” and “leadcalcium.”
Adding antimony and tin improves deep cycling but this increases water consumption and escalates the need to equalize. Calcium reduces self-discharge, but the positive lead-calcium plate has the side effect of growing due to grid oxidation when being over-charged. Modern lead acid batteries also make use of doping agents such as selenium, cadmium, tin and arsenic to lower the antimony and calcium content.
Lead acid is heavy and is less durable than nickel- and lithium-based systems when deep cycled. A full discharge causes strain and each discharge/charge cycle permanently robs the battery of a small amount of capacity. This loss is small while the battery is in good operating condition, but the fading increases once the performance drops to half the nominal capacity. This wear-down characteristic applies to all batteries in various degrees.
Depending on the depth of discharge, lead acid for deep-cycle applications provides 200 to 300 discharge/charge cycles. The primary reasons for its relatively short cycle life are grid corrosion on the positive electrode, depletion of the active material and expansion of the positive plates. This aging phenomenon is accelerated at elevated operating temperatures and when drawing high discharge currents. (See BU-804:How to Prolong Lead Acid Batteries)
Charging a lead acid battery is simple, but the correct voltage limits must be observed. Choosing a low voltage limit shelters the battery, but this produces poor performance and causes a buildup of sulfation on the negative plate. A high voltage limit improves performance but forms grid corrosion on the positive plate. While sulfation can be reversed if serviced in time, corrosion is permanent. (See BU-403: Charging Lead Acid)
Lead acid does not lend itself to fast charging and with most types, a full charge takes 14–16 hours. The battery must always be stored at full state-of-charge. Low charge causes sulfation, a condition that robs the battery of performance. Adding carbon on the negative electrode reduces this problem but this lowers the specific energy. (See BU-202: New Lead Acid Systems)
Lead acid has a moderate life span, but it is not subject to memory as nickel-based systems are, and the charge retention is best among rechargeable batteries. While NiCd loses approximately 40 percent of their stored energy in three months, lead acid self-discharges the same amount in one year. The lead acid battery works well at cold temperatures and is superior to lithium-ion when operating in subzero conditions. According to RWTH, Aachen, Germany (2018), the cost of the flooded lead acid is about $150 per kWh, one of the lowest in batteries.
Sealed Lead Acid
The first sealed, or maintenance-free, lead acid emerged in the mid-1970s. Engineers argued that the term “sealed lead acid” was a misnomer because no lead acid battery can be totally sealed. To control venting during stressful charge and rapid discharge, valves have been added that release gases if pressure builds up. Rather than submerging the plates in a liquid, the electrolyte is impregnated into a moistened separator, a design that resembles nickel- and lithium-based systems. This enables operating the battery in any physical orientation without leakage.
The sealed battery contains less electrolyte than the flooded type, hence the term “acid-starved.” Perhaps the most significant advantage of sealed lead acid is the ability to combine oxygen and hydrogen to create water and prevent dry out during cycling. The recombination occurs at a moderate pressure of 0.14 bar (2psi). The valve serves as a safety vent if the gas buildup rises. Repeated venting should be avoided as this will lead to an eventual dry-out. According to RWTH, Aachen, Germany (2018), the cost of VRLA is about $260 per kWh.
Several types of sealed lead acid have emerged and the most common are gel, also known as valve-regulated lead acid (VRLA), and absorbent glass mat (AGM). The gel cell contains a silica type gel that suspends the electrolyte in a paste. Smaller packs with capacities of up to 30Ah are often called SLA (sealed lead acid). Packaged in a plastic container, these batteries are used for small UPS, emergency lighting and wheelchairs. Because of low price, dependable service and low maintenance, the SLA remains the preferred choice for healthcare in hospitals and retirement homes. The larger VRLA is used as power backup for cellular repeater towers, Internet hubs, banks, hospitals, airports and more.
The AGM suspends the electrolyte in a specially designed glass mat. This offers several advantages to lead acid systems, including faster charging and instant high load currents on demand. AGM works best as a mid-range battery with capacities of 30 to 100Ah and is less suited for large systems, such as UPS. Typical uses are starter batteries for motorcycles, start-stop function for micro-hybrid cars, as well as marine and RV that need some cycling.
With cycling and age, the capacity of AGM fades gradually; gel, on the other hand, has a dome shaped performance curve and stays in the high performance range longer but then drops suddenly towards the end of life. AGM is more expensive than flooded, but is cheaper than gel. (Gel would be too expensive for start/stop use in cars.)
Unlike the flooded, the sealed lead acid battery is designed with a low over-voltage potential to prohibit the battery from reaching its gas-generating potential during charge. Excess charging causes gassing, venting and subsequent water depletion and dry-out. Consequently, gel, and in part also AGM, cannot be charged to their full potential and the charge voltage limit must be set lower than that of a flooded. This also applies to the float charge on full charge. In respect to charging, the gel and AGM are no direct replacements for the flooded type. If no designated charger is available for AGM with lower voltage settings, disconnect the charger after 24 hours of charge. This prevents gassing due to a float voltage that is set too high. (See BU-403: Charging Lead Acid)
The optimum operating temperature for a VRLA battery is 25°C (77°F); every 8°C (15°F) rise above this temperature threshold cuts battery life in half. (See BU-806a: How Heat and Loading affect Battery Life) Lead acid batteries are rated at a 5-hour (0.2C) and 20-hour (0.05C) discharge rate. The battery performs best when discharged slowly; the capacity readings are substantially higher at a slower discharge than at the 1C-rate. Lead acid can, however, deliver high pulse currents of several C if done for only a few seconds. This makes the lead acid well suited as a starter battery, also known as starter-light-ignition (SLI). The high lead content and the sulfuric acid make lead acid environmentally unfriendly.
Lead acid batteries are commonly classified into three usages: Automotive (starter or SLI), motive power (traction or deep cycle) and stationary (UPS).
Starter Batteries
The starter battery is designed to crank an engine with a momentary high-power load lasting a second or so. For its size, the battery is able to deliver high current but it cannot be deep-cycled. Starter batteries are rated with Ah or RS (reserve capacity) to indicate energy storage capability, as well as CCA (cold cranking amps) to signify the current a battery can deliver at cold temperature. SAE J537 specifies 30 seconds of discharge at –18°C (0°F) at the rated CCA ampere without the battery voltage dropping below 7.2 volts. RC reflects the runtime in minutes at a steady discharge of 25. (SAE stands for Society of Automotive Engineers.) See also BU-902a: How to Measure CCA.
Starter batteries have a very low internal resistance that is achieved by adding extra plates for maximum surface area (Figure 1). The plates are thin and the lead is applied in a sponge-like form that has the appearance of fine foam, expanding the surface area further. Plate thickness, which is important for a deep-cycle battery is less important because the discharge is short and the battery is recharged while driving; the emphasis is on power rather than capacity.
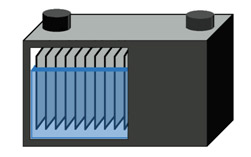
The starter battery has many thin plates in parallel to achieve low resistance with high surface area.
The starter battery does not allow deep cycling. Courtesy of Cadex
Deep-cycle Battery
The deep-cycle battery is built to provide continuous power for wheelchairs, golf cars, forklifts and more. This battery is built for maximum capacity and a reasonably high cycle count. This is achieved by making the lead plates thick (Figure 2). Although the battery is designed for cycling, full discharges still induce stress and the cycle count relates to the depth-of-discharge (DoD). Deep-cycle batteries are marked in Ah or minutes of runtime. The capacity is typically rated as a 5-hour and 20-hour discharge.
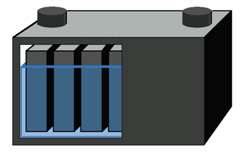
The deep-cycle battery has thick plates for improved cycling abilities.
The deep-cycle battery generally allows about 300 cycles. Courtesy of Cadex
A starter battery cannot be swapped with a deep-cycle battery or vice versa. While an inventive senior may be tempted to install a starter battery instead of the more expensive deep-cycle on his wheelchair to save money, the starter battery would not last because the thin sponge-like plates would quickly dissolve with repeated deep cycling.
There are combination starter/deep-cycle batteries available for trucks, buses, public safety and military vehicles, but these units are big and heavy. As a simple guideline, the heavier the battery is, the more lead it contains, and the longer it will last. Table 3 compares the typical life of starter and deep-cycle batteries when deep cycled.
Depth of Discharge | Starter Battery | Deep-Cycle Battery |
100% | 12–15 cycles | 150–200 cycles |
50% | 100–120 cycles | 400–500 cycles |
30% | 130–150 cycles | 1,000 and more cycles |
A discharge of 100% refers to a full discharge; 50% is half and 30% is a moderate discharge with 70% remaining.
Lead Acid or Li-ion in your Car?
Ever since Cadillac introduced the starter motor in 1912, lead acid batteries served well as battery of choice. Thomas Edison tried to replace lead acid with nickel-iron (NiFe), but lead acid prevailed because of its rugged and forgiving nature, as well as low cost. Now the lead acid serving as starter battery in vehicles is being challenged by Li-ion.
Figure 4 illustrates the characteristics of lead acid and Li-ion. Both chemistries perform similarly in cold cranking. Lead acid is slightly better in W/kg, but Li-ion delivers large improvements in cycle life, better specific energy in Wh/kg and good dynamic charge acceptance. Where Li-ion falls short is high cost per kWh, complex recycling and less stellar safety record than lead acid.
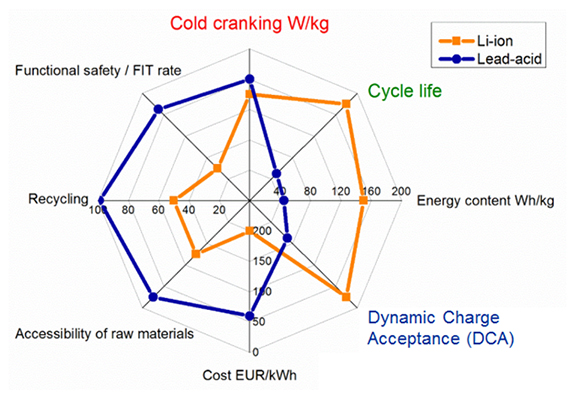
Lead acid maintains a strong lead in starter battery. Credit goes to good cold temperature performance, low cost, good safety record and ease of recycling.[1]
Lead is toxic and environmentalists would like to replace the lead acid battery with an alternative chemistry. Europe succeeded in keeping NiCd out of consumer products, and similar efforts are being made with the starter battery. The choices are NiMH and Li-ion, but the price is too high and low temperature performance is poor. With a 99 percent recycling rate, the lead acid battery poses little environmental hazard and will likely continue to be the battery of choice.
Table 5 lists advantages and limitations of common lead acid batteries in use today. The table does not include the new lead acid chemistries. (See also BU-202: New Lead Acid Systems)
| Advantages |
|
| Limitations |
|
Dry systems have advantages over flooded but are less rugged.
References
[1] Source: Johnson Control

Comments
We would like to know if you carry the below item:
*GNB MARATHON VALVE REGULATED LEAD ACID (VRLA) M12V 200FTX
QTY: 80UNITS
I will appreciate a reply at your earliest convenience.
Best Regards
Abraham Phillips
General Manager
Jones Memorial Hospital
191 North Main Street, Wellsville, NY 14895
Abraham@urmcrrochester.com
315-633-4859
Hello, thank you for your good site
Are the batteries made in European countries different in terms of the charging end point?
can you guide me
For example, Italian batteries are different from German batteries in terms of the charging voltage. I think the cut-off voltage of Italian batteries is 2.65 volts for each cell.
But German batteries are 2.45 volts for each cell
Is what I said correct?
Please guide me
Looking for comments from the previous website?
Comments from the previous website are not compatible with our new commenting system but we have preserved them so our users can still reference and make use the information in them.

I like the topic