Pioneering work of the lithium battery began in 1912 under G.N. Lewis, but it was not until the early 1970s that the first non-rechargeable lithium batteries became commercially available. Attempts to develop rechargeable lithium batteries followed in the 1980s but failed because of instabilities in the metallic lithium used as anode material. (The metal-lithium battery uses lithium as anode; Li-ion uses graphite as anode and active materials in the cathode.)
Lithium is the lightest of all metals, has the greatest electrochemical potential and provides the largest specific energy per weight. Rechargeable batteries with lithium metal on the anode could provide extraordinarily high energy densities; however, it was discovered in the mid-1980s that cycling produced unwanted dendrites on the anode. These growth particles penetrate the separator and cause an electrical short. The cell temperature would rise quickly and approach the melting point of lithium, causing thermal runaway, also known as “venting with flame.” A large number of rechargeable metallic lithium batteries sent to Japan were recalled in 1991 after a battery in a mobile phone released flaming gases and inflicted burns to a man’s face.
The inherent instability of lithium metal, especially during charging, shifted research to a non-metallic solution using lithium ions. In 1991, Sony commercialized the first Li ion, and today this chemistry has become the most promising and fastest growing battery on the market. Although lower in specific energy than lithium-metal, Li ion is safe, provided the voltage and currents limits are being respected. (See BU-304a: Safety Concerns with Li-ion)
Credit for inventing the lithium-cobalt-oxide battery should go to John B. Goodenough (1922). It is said that during the developments, a graduate student employed by Nippon Telephone & Telegraph (NTT) worked with Goodenough in the USA. Shortly after the breakthrough, the student traveled back to Japan, taking the discovery with him. Then in 1991, Sony announced an international patent on a lithium-cobalt-oxide cathode. Years of litigation ensued, but Sony was able to keep the patent and Goodenough received nothing for his efforts. In recognition of the contributions made in Li-ion developments, the U.S. National Academy of Engineering awarded Goodenough and other contributors the Charles Stark Draper Prize in 2014. In 2015, Israel awarded Goodenough a $1 million prize, which he will donate to the Texas Materials Institute to assist in materials research.
The key to the superior specific energy is the high cell voltage of 3.60V. Improvements in the active materials and electrolytes have the potential to further boost the energy density. Load characteristics are good and the flat discharge curve offers effective utilization of the stored energy in a desirable and flat voltage spectrum of 3.70–2.80V/cell.
In 1994, the cost to manufacture Li-ion in the 18650 cylindrical cell was over US$10 and the capacity was 1,100mAh. In 2001, the price dropped to below $3 while the capacity rose to 1,900mAh. Today, high energy-dense 18650 cells deliver over 3,000mAh and the costs are dropping. Cost reduction, increased specific energy and the absence of toxic material paved the road to make Li-ion the universally accepted battery for portable applications, heavy industries, electric powertrains and satellites. The 18650 measures 18mm in diameter and 65mm in length. (See BU-301: A look at Old and New Battery Packaging)
Li-ion is a low-maintenance battery, an advantage that most other chemistries cannot claim. The battery has no memory and does not need exercising (deliberate full discharge) to keep it in good shape. Self-discharge is less than half that of nickel-based systems and this helps the fuel gauge applications. The nominal cell voltage of 3.60V can directly power mobile phones, tablets and digital cameras, offering simplifications and cost reductions over multi-cell designs. The drawbacks are the need for protection circuits to prevent abuse, as well as high price.
Types of Lithium-ion Batteries
Lithium-ion uses a cathode (positive electrode), an anode (negative electrode) and electrolyte as conductor. (The anode of a discharging battery is negative and the cathode positive (see BU-104b: Battery Building Blocks). The cathode is metal oxide and the anode consists of porous carbon. During discharge, the ions flow from the anode to the cathode through the electrolyte and separator; charge reverses the direction and the ions flow from the cathode to the anode. Figure 1 illustrates the process.
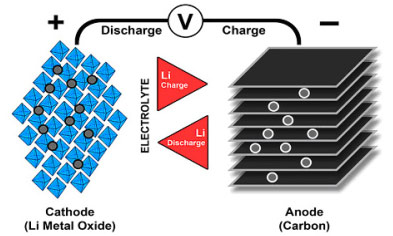
When the cell charges and discharges, ions shuttle between cathode (positive electrode) and anode (negative electrode). On discharge, the anode undergoes oxidation, or loss of electrons, and the cathode sees a reduction, or a gain of electrons. Charge reverses the movement.
Li ion batteries come in many varieties but all have one thing in common – the “lithium-ion” catchword. Although strikingly similar at first glance, these batteries vary in performance and the choice of active materials gives them unique personalities. (See BU-205: Types of Li-ion-ion)
Sony’s original lithium-ion battery used coke as the anode (coal product). Since 1997, most Li ion manufacturers, including Sony, shifted to graphite to attain a flatter discharge curve. Graphite is a form of carbon that has long-term cycle stability and is used in lead pencils. It is the most common carbon material, followed by hard and soft carbons. Nanotube carbons have not yet found commercial use in Li-ion as they tend to entangle and affect performance. A future material that promises to enhance the performance of Li-ion is graphene.
Figure 2 illustrates the voltage discharge curve of a modern Li-ion with graphite anode and the early coke version.
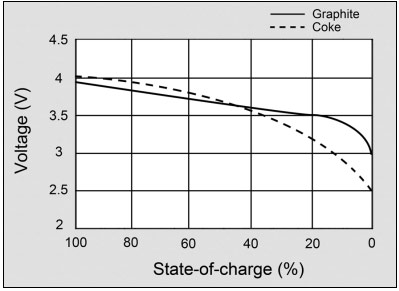
A battery should have a flat voltage curve in the usable discharge range. The modern graphite anode does this better than the early coke version. Courtesy of Cadex
Several additives have been tried, including silicon-based alloys, to enhance the performance of the graphite anode. It takes six carbon (graphite) atoms to bind to a single lithium ion; a single silicon atom can bind to four lithium ions. This means that the silicon anode could theoretically store over 10 times the energy of graphite, but expansion of the anode during charge is a problem. Pure silicone anodes are therefore not practical and only 3–5 percent of silicon is typically added to the anode of a silicon-based to achieve good cycle life.
Using nano-structured lithium-titanate as an anode additive shows promising cycle life, good load capabilities, excellent low-temperature performance and superior safety, but the specific energy is low and the cost is high.
Experimenting with cathode and anode material allows manufacturers to strengthen intrinsic qualities, but one enhancement may compromise another. The so-called “Energy Cell” optimizes the specific energy (capacity) to achieve long runtimes but at lower specific power; the “Power Cell” offers exceptional specific power but at lower capacity. The “Hybrid Cell” is a compromise and offers a little bit of both. (More on BU-501: Basics About Discharging)
Manufacturers can attain a high specific energy and low cost relatively easily by adding nickel in lieu of the more expensive cobalt, but this makes the cell less stable. While a start-up company may focus on high specific energy and low price to gain quick market acceptance, safety and durability cannot be compromised. Reputable manufacturers place high integrity on safety and longevity. Table 3 summarizes the advantages and limitations of Li-ion.
Most Li-ion batteries share a similar design consisting of a metal oxide positive electrode (cathode) that is coated onto an aluminum current collector, a negative electrode (anode) made from carbon/graphite coated on a copper current collector, a separator and electrolyte made of lithium salt in an organic solvent. Table 3 summarizes the advantages and limitations of Li-ion.
| Advantages |
|
| Limitations |
|

Looking for comments from the previous website?
Comments from the previous website are not compatible with our new commenting system but we have preserved them so our users can still reference and make use the information in them.

Anions (electrons) flow to the positively charged anode. In cathode ray tube (tv) a beam of electrons are drawn a from a glowing hot anion towards the cathode (positive).