Abstract
Lithium-ion batteries do occasionally overheat and catch fire, and this is a large concern in industry. Regulatory approvals are tough, but once accepted and rubberstamped; officials wash their hands and put the responsibility for care in the hands of the user. Approval procedures are mainly directed towards birth-to-graduation; few regularity requirements exist after the battery has been approved as part of workforce to retirement. While this works well for the more benign lead- and nickel-based batteries; lithium-ion has definite red lines that must be respected.
This article touches on the approval process and proposes methods to monitor Li-ion batteries in the field to detect anomalies that may develop under environmental stresses. All batteries age but changes in characteristics are especially troublesome with Li-ion. One way to observe anomalies is including diagnostic features in chargers and battery-operated devices, a method that will improve safety without added logistics. The article also addresses the choice of Li-ion to meet load profile and environmental exposures. Such considerations may one day play a role in the approval process to assure batteries operate within a safe bandwidth.
Safety Testing and Monitoring
Manufacturers are ultimately responsible for the safety of a battery. UN/DOT 38.3 requires that Li-ion cells be tested for air worthiness to endure altitude simulation, thermal, vibration, shock short circuit, impact, overcharge and forced discharge checks. These tests are done on new or near new Li-ion cells, but not packs that may be past their prime. The safety verifications in the workforce seem to be overlooked; all efforts go to before approval; the after approval moves beyond reach.
The before approval resembles the life of students receiving academic knowledge to prepare for a career with examination to test the understanding before graduation; the after approval represents the workforce where users are on their own, often with little guidance. Rules become vague. Having to depend on a power source that also diminishes capacity with each cycle prompts the user to ask: “How often should I test the battery? How much spare capacity is enough? At what capacity should I replace the battery?”
In spite of rigorous testing, Li-ion fires are being reported, and these infractions are of serious concern to healthcare, defense and communications. With more Li-ion also being deployed for large scale uses, the question is asked, “should more be done to assure continued safety once the battery is past its prime?” Instead of placing all efforts in the before approval phase, a maintenance-based system would monitor the battery in the field and advise the user of emerging defects that could compromise safety. The Li-ion battery is unique in that it can hide a defect while continuing to deliver full performance. A faulty building or a corroded bridge reacts differently in that structures display visible stress marks that enable safety measures; Li-ion does not give such prior warning.
The ongoing safety of Li-ion batteries can be summarized into three categories:
- Product Certification. This endorsement assures safety of a battery as part of birth-to-graduation. The approval provides a snapshot of a new battery; however, performance degradation by wear-and-tear and extreme usage cannot be predicted accurately.
- Field Diagnostics represents workforce-to-retirement to observe anomalies while the battery is in use. Improvements are possible by adding monitoring functions into a charger or a device by using advanced algorithms that will warn the user of a pending condition.
- Harm Reduction. Not all failures are avoidable and a battery should be designed to minimize damage if a thermal event occurs by preventing propagation from one cell to another.
Product certification gives the green light to release a Li-ion cell into the market; it also enables air shipment. However, maintenance issues appear only once a product has been in use for a while. Batteries should receive a similar treatment as a critical part in an aircraft or machine in which wear and tear falls under guidelines. This is especially important when batteries are exposed to stressful conditions that include heat, ultra-rapid charging, charging below freezing, harsh loading and vibration.
To stay within the red line, the choice of Li-ion is important in the design process. The Energy Cell provides high specific energy but delivers moderate loading currents and requires long charge times. The Power Cell is reserved for high load current that also enables faster charging but it has reduced capacity. The need to tailor a battery to specific applications was less common with nickel-cadmium (NiCd) systems. One type served most uses.
A compromised Li-ion in fail-mode is more critical than lead- and nickel-based systems. Separator damage in Li-ion must be taken seriously as most fires are caused by a separator failure that can lead to an electrical short. NiCds are more forgiving in this respect. The separator can get so marred by high cycle count and crystalline formation (memory) that the battery self-discharges in 8 hours.
High self-discharge in Li-ion can also occur when dendrites form as a result of ultra-fast charging, harsh loading or sub-freezing charging. Storing a Li-ion cell in low state-of-charge is also known to grow dendrites. One of the biggest concerns of Li-ion manufacturers, however, is foreign particles converging on one spot. Improvements have been made but they report that complex assembly techniques make the elimination of all metallic dust a challenge. Cells with ultra-thin separators of only 20µm or less (20-thousandth of an mm) are more susceptible to impurities than the older designs.
A compromised separator in a Li-ion cell may only causes a slight elevation of self-discharge. For most cells, this condition remains stable, but in rare cases, the fault line can develop into a sizable current flowing between the electrodes in the cell. The spot may heat up and weaken the separator further until it develops into an electrical short. An analogy is a small water leak in a faulty hydro dam that can turn into a torrent and takes the structure down. The temperature of a shorted Li-ion can quickly reach 500°C (932°F), at which point the cell catches fire or explodes. A thermal runaway is known as “venting with flame.” Once in progress, the event cannot be stopped and needs to burn out.
Battery manufacturers share cleanliness standards with the semiconductor industry. Chip makers have spent millions of dollars to reduce particles that can lead to the loss of wafers. The world’s best cleanrooms are ISO 4 (Class 10), meaning no more than 10,000 particles larger than 0.1 micrometer (µm) are present. Even at this low level, particles still affect semiconductor wafers. Batteries are often made in less stringently controlled cleanrooms and cleanliness may need to improve to be equal or better than that of semiconductor manufacturing.
A tier-1 supplier acknowledges that lithium-ion batteries have a fire risk. Their suggested precaution includes current limiting in a parallel configuration to render a shorted cell harmless, lowering charge voltages when the battery capacity fades, providing traceable lot numbers down to cell and pack level, building a pack that inhibits propagation from cell to cell in case of fire, and enabling exhaust gases to escape while preventing fire from leaking outside the pack.
The tier-1 supplier also reveals that cycling increases the pressure to the electrodes. The anode thickens with use, which further intensifies pressure on the separator. With foreign materials that cannot be fully avoided, the tier-1 supplier confirms that normal use of Li-ion can lead to an electrical short in isolated cases. Because of customer requests for higher capacities and maintaining the required safety level at all time, the tier-1 supplier recommends reducing the charge voltage to adjust for capacity fade as follows:
- 100–70% capacity apply the full 4.20V/cell (4.35V/cell or higher for some cells)
- 70–50% capacity reduce the voltage limit to 4.15V/cell (4.35V/cell to 4.25V/cell)
- 50% and lower capacity, decrease the voltage to 3.8V/cell.
The tier-1 supplier also suggests reducing the charge voltage by 0.1V in healthcare for critical and disabled patients. Figures 1 and 2 illustrate the intrinsic changes of a Li-ion cell as a function of aging that can lead to premature failure.
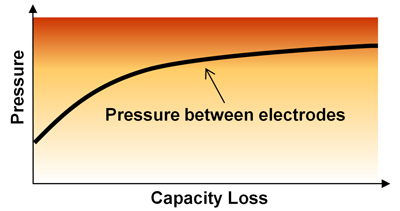
Increased pressure raises the risk of internal short if foreign materials are present. A cylindrical cell maintains correct size. Expansion allowance must be made for prismatic and pouch cells.
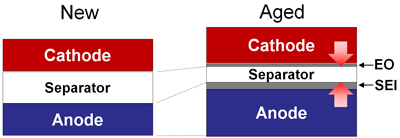
Increased pressure reduces the thickness of the separator. Aging also grows the solid electrolyte interface (SEI) on the anode and electrolyte oxidation (EO) on the cathode.
Courtesy of Panasonic/Cadex
The failure rate of Li-ion has improved and is now better than one-in-10 million. Credit goes to diligent battery manufacturers that take safety seriously. No other battery failure draws more media attention than a Li-ion fire. All energy storage devices carry risk and the public was alarmed in the 1800s when steam engines exploded and people got hurt. Carrying highly flammable gasoline in cars was a hot topic in the early 1900s. Thomas Edison had good reasons to promote the electric car in the early 1900s as being a better option. He promoted nickel-iron in favor of lead acid, not lithium-ion.
There are two types of Li-ion failures. One involves a design or manufacturing flaw in which the failure repeats itself at a predictable rate and often involves a recall; the Samsung Galaxy 7 smartphones in 2016 and the Sony laptop batteries in 2006 are examples. The most difficult failures are random events. These may be caused by repeated charging at sub-freezing temperature, harsh loading or excessive vibration. It can also be a fluke similar to being hit by a meteor.
Cadex is performing lab tests to measure the self-discharge rates between good Li-ion cells and those that have been stressed. The objective is to develop a failure detection method, assuming that a thermal event is proceeded by high self-discharge. To our knowledge, no formal research has been carried out on this topic. If elevated self-discharge can be identified on a compromised Li-ion cell, algorithms can be developed that issue a CAUTION message if the battery needs replacement and a DANGER if the pack reaches the threat of a thermal event.
Summary and Proposed Solution
Li-ion is one of the best battery systems but it is unforgiving in fail-mode. Maintenance-based safety features may be needed for some systems as part of after approval to enable safe operation of Li-ion from workforce-to-retirement under all conditions. Traditional certification was sufficient to approve lead- and nickel-based batteries because these systems reach end-of-life by loss of performance. Although fires are also reported with non-lithium-based batteries, they are less frequent per watt-hour and are more manageable.
Many Li-ion batteries keep performing even when a fault develops. An analogy is an aging steam engine that still delivers full power with a boiler that no longer meets safety requirements. Concerns also apply to aging Li-ion installations in Energy Storage Systems and boats. The aging syndrome may not be fully understood yet as most these systems are new. We ask the question: “will capacity or safety determine the end-of-life?’
Chargers with diagnostic features that advise the user of a pending battery defect are possible. These chargers will perform quality control by predicting when a battery needs replacing based on capacity estimations and identifying anomalies.
Software based on advanced machine learning (AML) is in development that can be added to a charger or portable device to provide monitoring functions at an economical price. These systems go beyond voltage, current and coulomb-counting, but examine the health of the chemical battery. AML is also used in voice and image recognition, as well as the self-driving car. Besides making the battery safer, AML estimates battery capacity during charge to make performance data transparent to the user. It’s connecting dots by utilizing existing technologies that will make the battery a safer, more reliable and environmentally friendly product.
About the Author
Isidor Buchmann is the founder and CEO of Cadex Electronics Inc. For three decades, Buchmann has studied the behavior of rechargeable batteries in practical, everyday applications, has written award-winning articles including the best-selling book “Batteries in a Portable World,” now in its fourth edition. Cadex specializes in the design and manufacturing of battery chargers, analyzers and monitoring devices. For more information on batteries, visit www.batteryuniversity.com; product information is on www.cadex.com.

