A fuel cell is an electrochemical device that combines hydrogen fuel with oxygen to produce electricity, heat and water. The fuel cell is similar to a battery in that an electrochemical reaction occurs as long as fuel is available. Hydrogen is stored in a pressurized container and oxygen is taken from the air. Because of the absence of combustion, there are no harmful emissions, and the only by-product is pure water. So pure is the water emitted from the proton exchange membrane fuel cell (PEMFC) that visitors to Vancouver’s Ballard Power Systems were served hot tea made from this clean water.
Fundamentally, a fuel cell is electrolysis in reverse, using two electrodes separated by an electrolyte. The anode (negative electrode) receives hydrogen and the cathode (positive electrode) collects oxygen. A catalyst at the anode separates hydrogen into positively charged hydrogen ions and electrons. The oxygen is ionized and migrates across the electrolyte to the anodic compartment, where it combines with hydrogen. A single fuel cell produces 0.6–0.8V under load. To obtain higher voltages, several cells are connected in series. Figure 1 illustrates the concept of a fuel cell.
.jpg)
The anode (negative electrode) receives the hydrogen and the cathode (positive electrode) collects the oxygen.
Fuel cell technology is twice as efficient as combustion in turning carbon fuel to energy. Hydrogen, the simplest chemical element (one proton and one electron), is plentiful and exceptionally clean as a fuel. Hydrogen makes up 90 percent of the universe and is the third most abundant element on the earth’s surface. Such a wealth of fuel would provide an almost unlimited pool of clean energy at relatively low cost. But there is a hitch.
With most fuels, hydrogen is bonded to other substances and “unleashing” the gas takes energy. In terms of net calorific value (NCV), hydrogen is more costly to produce than gasoline. Some say that hydrogen is nearly energy neutral, meaning that it takes as much energy to produce as it delivers at the end destination. (See BU-1007: Net Calorific Value)
Storage of hydrogen poses a further disadvantage. Pressurized hydrogen requires heavy steel tanks, and the NCV by volume is about 24 times lower than a liquid petroleum product. In liquid form, which is much denser, hydrogen needs extensive insulation for cold storage.
Hydrogen can also be produced with a reformer by means of extraction from an existing fuel, such as methanol, propane, butane or natural gas. Converting fossil fuel into pure hydrogen releases some leftover carbon, but this is 90 percent less harmful than what comes from the tailpipe of a car. Carrying a reformer would add weight to the vehicle and increase its cost; reformers are also sluggish. The net benefit of hydrogen conversion is in question because it does not solve the energy problem.
Sir William Grove, a Welsh judge and gentleman scientist, developed the fuel cell concept in 1839, but the invention never took off. This was during the development of the internal combustion engine (ICE) that showed promising results. It was not until the 1960s that the fuel cell was put to practical use during the Gemini space program. NASA preferred this clean power source to nuclear or solar power. The alkaline fuel cell system that was chosen generated electricity and produced drinking water for the astronauts.
High material costs made the fuel cell prohibitive for commercial use. The fuel cell core (stack) is expensive and has a limited life span. Burning fossil fuel in a combustion engine is the simplest and most effective means to harness energy, but it pollutes.
High cost did not discourage the late Karl Kordesch, the co-inventor of the alkaline battery, from converting his car to an alkaline fuel cell in the early 1970s. He mounted the hydrogen tank on the roof and placed the fuel cell and backup batteries in the trunk. According to Kordesch, there was enough room for
ilar to a battery. Stack replacement is a major expense.
Alkaline Fuel Cell (AFC)
The alkaline fuel cell has become the preferred technology for aerospace, including the space shuttle. Manufacturing and operating costs are low, especially for the stack. While the separator for the PEM costs between $800 and $1,100 per square meter, the same material for the alkaline system is almost negligible. (The separator for a lead acid battery costs $5 per square meter.) Water management is simple and does not need compressors and other peripherals; efficiency is in the 60 percent range. A negative is that the AFC is larger in physical size than the PEM and needs pure oxygen and hydrogen as fuels. The amount of carbon dioxide present in a polluted city can poison the stack and this limits the AFC to specialized applications.
Solid Oxide Fuel Cell (SOFC)
Electric utilities use three types of fuel cells, which are molten carbonate, phosphoric acid and solid oxide fuel cells. Among these choices, the solid oxide (SOFC) is the least developed, but it has received renewed attention because of breakthroughs in cell material and stack design. Rather than operating at the very high operating temperature of 800–1,000°C (1,472–1,832°F), a new generation of ceramic material has brought the core down to a more manageable 500–600°C (932–1,112°F). This allows the use of conventional stainless steel rather than expensive ceramics for auxiliary parts.
High temperature allows direct extraction of hydrogen from natural gas through a catalytic reforming process. Carbon monoxide, a contaminant for the PEM, is a fuel for the SOFC. Being able to accept carbon-based fuels without a designated reformer and delivering high efficiency poses significant advantages for this type of fuel cell. Cogeneration by running steam generators from the heat by-product raises the SOFC to 60 percent efficiency, one of the highest among fuel cells. As a negative, high stack temperature requires exotic materials for the core that adds to manufacturing costs and reduces longevity.
Direct Methanol Fuel Cell (DMFC)
Portable fuel cells have gained attention and the most promising development is the direct methanol fuel cell. This small unit is inexpensive to manufacture, convenient to use and does not require pressurized hydrogen gas. The DMFC has good electrochemical performance and refilling is done by squirting in liquid or replacing the cartridge. This enables continued operation without downtime.
Manufactures admit that a direct battery replacement by the fuel cell is years away. To bridge the gap, the micro fuel cell serves as a charger to provide continuous operation for the onboard battery. Furthermore, methanol is toxic and flammable, and there are limitations to how much fuel passengers can carry on an aircraft. In 2008 the Department of Transportation issued a ruling to permit passengers and crew to carry an approved fuel cell with an installed methanol cartridge and up to two additional spare cartridges of 200 ml (6.76 fl oz). This provision does not yet extend to bottled hydrogen.
Figure 2 shows a micro fuel cell by Toshiba and Figure 3 demonstrates refueling with methanol that is 99.5 percent pure.
.jpg) This prototype micro fuel cell is capable of providing 300mW of continuous power. | .jpg) The fuel in a 10ml tank is 99.5 percent pure methanol. |
Improvements are being made, and Toshiba unveiled prototype fuel cells for laptops and other applications generating 20 to 100 watts. The units are compact and the specific energy is comparable with that of a NiCd battery. Meanwhile, Panasonic claims to have doubled the power output with a similar size, specifying a calendar life of 5,000 hours if the fuel cell is used intermittently for 8 hours per day. The low longevity of these fuel cells has been an issue to be reckoned with.
Attempts are being made with small fuel cells running on stored hydrogen. Increased efficiency and smaller size are the advantages of pure hydrogen over methanol. These miniature systems have no pumps and fans and are totally silent. A 21cc cartridge is said to provide the equivalent energy of about 10 AA alkaline batteries with a runtime between refueling of 20 hours. This lends itself to portable computing, wireless communications and flashlights for the bicycle lone rider.
Military and recreational users are also experimenting with the miniature fuel cell. Figure 4 illustrates a portable fuel cell made by SFC Smart Fuel Cell. The EFOY fuel cell comes in different capacities that ranges from 600 to 2,160 watt-hours per day.
.jpg)
The fuel cell converts hydrogen and oxygen to electricity and clean water is the only by-product. Fuel cells can be used indoors as an electricity generator.
Table 5 describes the applications and summarizes the advantages and limitations of common fuel cells. The table also includes the Molten Carbonate (MCFC) and Phosphoric Acid (PAFC), classic fuel cell systems that have been around for a while and have unique advantages.
Type of fuel cell |
Applications |
Core temp. efficiency | Advantages | Limitations |
Proton Exchange Membrane
(PEMFC) |
Portable, stationary and automotive | 50–100°C; 80°C typical; 35–60% efficient | Compact design, long operating life, quick start-up, well developed | Expensive catalyst; needs chemical grade fuel; complex heat and water control |
Alkaline
(AFC) |
Space, military, submarines, transport | 90–100°C; 60% efficient | Low parts and, operation costs; no compressor; fast cathode kinetics | Large size; sensitive to hydrogen and oxygen impurities |
Molten Carbonate
(MCFC) |
Large power generation | 600–700°C; 45–50% efficient | High efficiency, flexible to fuel, co-generation | High heat causes corrosion, long startup, short life |
Phosphoric Acid
(PAFC) |
Medium to large power generation | 150–200°C; 40% efficient | Good tolerance to fuel impurities; co-generation | Low efficiency; limited service life; expensive catalyst |
Solid Oxide
(SOFC) |
Medium to large power generation | 700–1000°C; 60% efficient | Lenient to fuels; can use natural gas, high efficient | High heat causes corrosion, long startup, short life |
Direct Methanol
(DMFC) |
Portable, mobile and stationary use | 40–60°C; 20% efficient | Compact; feeds on methanol; no compressor | Complex stack; slow response; low efficiency |
Fuel cell developments have been gradual; the specific power is low and a direct battery replacement may never be feasible.
Developments
Limitations involve slow start-up times, low power output, sluggish response on power demand, poor loading capabilities, narrow power bandwidth, short service life and high cost. Similar to batteries, the performance of all fuel cells degrades with age, and the stack gradually loses efficiency. Such performance losses are much less apparent with the ICE.
Fuel cells below 1kW are normally non-pressurized and only use a fan to aid in oxygen supply; fuel cells above 1kW are pressurized and include a compressor that lowers efficiency and the system can get rather noisy. The relatively high internal resistance of fuel cells poses a further challenge. Each cell of a stack produces about 1 volt in open circuit; a heavy load causes a notable voltage drop. Similar to the battery, the power bandwidth decreases with age. Individual cells in the stack are also known to cause failures and contaminants are large contributors. Figure 6 illustrates the voltage and power bandwidth as a function of load.
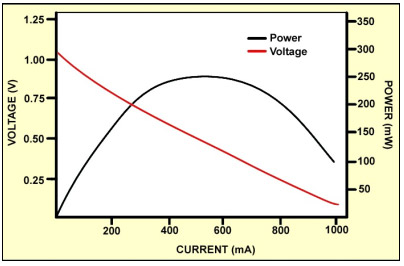
The power band is limited to between 300 and 800mA[3]
Fuel cells operate best at a 30 percent load factor; higher loads reduce efficiency. This and poor throttle response place the fuel cell into a support mode or a charger to keep batteries charged. A stand-alone power source, as the developers had hoped, has not materialized.
Longevity
Longevity results are becoming available for PEM fuel cells after 20 years of operation in busses. A typical service life running in moderate climates like California and England is 32,000 hours before the power drops to 80%, which denotes end-of-life. Many of the outstanding problems have been resolved with electronic monitoring.
Fuel cell powered buses and trucks for long routes are superior to battery power only because of range. Refueling is under 10 minutes, similar to diesel buses. The infrastructure required for refueling with hydrogen is said to be cheaper and more compact compared to recharging batteries in large vehicles. More information is on: BU-1005: Does the Fuel Cell-powered Vehicle have a Future?
Paradox of the fuel cell
The fuel cell enjoyed the height of popularity in the 1990s, when scientists and stock promoters envisioned a world running on a clean and inexhaustible resource — hydrogen. They predicted that cars would run on fuel cells, and that household electricity would also be generated by fuel cells. The stock prices skyrocketed but marginal performance, high manufacturing costs and limited service life moderated the hydrogen dream.
It was said that the fuel cell would transform the world as the microprocessor did in the 1970s. A clean and inexhaustible source of energy would become available that would solve the environmental concerns of burning fossil fuel. From 1999 through 2001, more than 2,000 organizations got actively involved in fuel cell development, and four of the largest public fuel cell companies in North America raised over a billion US dollars in public stock offerings. What went wrong?
Hydrogen is not a source of energy per se but a medium to transport and store energy similar to electricity that charges a battery. To envision “burning an endless supply of hydrogen,” the fuel must first be produced, because hydrogen cannot be pumped from the earth as is possible with oil. While fossil fuel lends itself well to producing hydrogen, taking this valuable fuel to unleash hydrogen makes little sense when it costs as much or more for extraction as burning it directly. The only benefit is reduced greenhouse gases.
Just as the attempt to fly airplanes on steam failed in the mid-1800s, it is conceivable that the fuel cell will never be the powerhouse scientists had hoped for. But there is renewed interest in the automotive field in Japan. Fuel cells are replacing battery banks and diesel generators in office buildings as they can be installed in tight storage places with minimal maintenance and without the need for exhaust. Fuel cells allow continuous and pollution-free operation of forklifts in warehouses, whereas 40M fuel cells generate clean electricity in remote locations. The ultimate dream is propelling vehicles with the clean fuel cell.
Fuel cells may one day taxi airplanes with electric wheel hub motors. This would lower pollution and save up to 4 percent fuel by not running the jet engines. Water produced from the fuel cell while charging the batteries could serve as on-board drinking water; regenerative braking could further assist in charging the batteries and supercapacitors for fast charge acceptance. The ultimate dream is propelling airplanes and vehicles with the clean fuel cell.
References
[1] Source: US Department of Energy, office of Energy Efficiency and Renewable Energy
[2] Courtesy of SFC Smart Fuel Cell AG (2010)
[3] Courtesy of Cadex

Looking for comments from the previous website?
Comments from the previous website are not compatible with our new commenting system but we have preserved them so our users can still reference and make use the information in them.

Please add one more chapter on Fuel Cell materials, building blocks, reaction and Fuel cell size calculations vs voltage and power output.