Ever since Cadillac invented the starter motor in 1912, car mechanics have explored ways to measure cold cranking amps (CCA). CCA measurements assure that the battery has sufficient power to crank the engine, especially when cold. Typical CCA readings for a car range from 350 to 600A and higher for trucks. SAE J537 specifies that a battery with a CCA reading of 500A can deliver 500A at –18°C (0°F) for 30 seconds without dropping below 7.2 volts.

Source: BMW
CCA cannot be “measured,” but it can be “estimated” and the process can take a week per battery. A full CCA test is tedious and is seldom done. To test CCA, apply different discharge currents to see which amperage keeps the battery above a set voltage while cold. Table 1 illustrates the test procedures according to SAE J537, IEC and DIN. The methods are similar and only differ in the length of discharge and the cut-off voltages.
| SAE J537 CCA test | IEC CCA test | DIN CCA test |
|---|---|---|
| Fully charge battery according to SAE J537 and cool to -18°C (0°F) for 24 hours. While at subfreezing temperature, apply a discharge current equal to the specified CCA. (500 CCA battery discharges at 500A.) To pass, the voltage must stay above 7.2V (1.2V/cell) for 30 seconds. | Fully charge battery according to SAE J537 and cool to -18°C (0°F) for 24 hours. While at subfreezing temperature, apply a discharge current equal to the specified CCA. (500 CCA battery discharges at 500A.) To pass, the voltage must stay above 8.4V for 60 seconds. | Fully charge battery according to SAE J537 and cool to -18°C (0°F) for 24 hours. While at subfreezing temperature, apply a discharge current equal to the specified CCA. (500 CCA battery discharges at 500A.) To pass, the voltage must stay above 9V for 30s and 6V for 150s. |
The methods differ in the length of discharge and the cut-off voltages. (See SAE J537 in Glossary)
A variety of battery testers have emerged that read CCA. Since current flow relates to ohmic value, most CCA testers measure the internal battery resistance. To test the CCA with a carbon pile, a battery that must have an SoC of 70 to 100 percent. It is then loaded with half the rated CCA for 15 seconds at a temperature of 10º C (50º F) and higher. As an example, a 500 CCA battery will discharge at 250A for 15s, and the battery passes if the voltage stays above 9.6V. Colder temperatures will cause the voltage to drop further. The carbon pile simulates real-life cranking conditions while observing the voltage, but this method cannot estimate capacity.
Mechanics prefer small sizes and device manufactures have developed handheld testers, which induce a momentary high-current pulse that corresponds to the entered CCA value. Ohm’s law calculates the internal resistance on hand of the induced voltage drop, and the device provides a CCA-equivalent reading. This test method is fast and convenient, but it does not estimate capacity.
The AC conductance method reads CCA by injecting a single frequency of 80–90 hertz to the battery. These non-invasive units are small and stay cool during the test, but the battery should have a SoC of 70 percent and higher. As with other resistance-based test methods, AC conductance cannot read capacity.
Battery scientists predict that future battery diagnostics lie in EIS by combining the test results of the Randles model with complex modeling to estimate CCA and capacity. Called multi-model electrochemical impedance spectroscopy, the Spectro™-series battery rapid-testers (by Cadex) are the first to utilize the EIS technology(See BU-904: How to Measure Capacity)
Accuracy has always been in question and CCA is especially difficult to verify as the readings are affected by SoC, temperature and and other factors. Figure 2 compares CCA readings taken with AC conductance and the Spectro™ technology at different SoC. Both measurements decline with lower SoC; however Spectro™ is affected less than AC conductance. Since many batteries hover at about 70 percent when the car is brought in for service, the CCA readings between the two methods will appear similar.
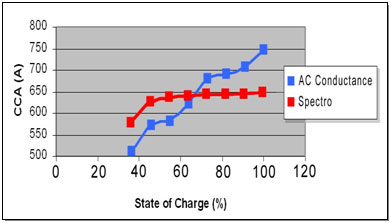
The Spectro CA-12 provides stable CCA readings between a SoC of 100 and 40% (red); the values on AC Conductance drop rapidly with SoC (blue).
Figure 3 illustrates CCA readings as a function of SoC and battery performance. The CCA of Battery A, with a capacity of 100 percent, stays steady down to an SoC of 10 percent; Battery B, with 37 percent capacity, starts to show instabilities at an SoC of about 40 percent; and Battery C, with only 22 percent capacity, provides uncertain results. The test clearly demonstrates that battery state-of-health affects the readings. A good battery provides strong symptoms with good accuracy; the readings from a weak battery are muddled and the results are less consistent.
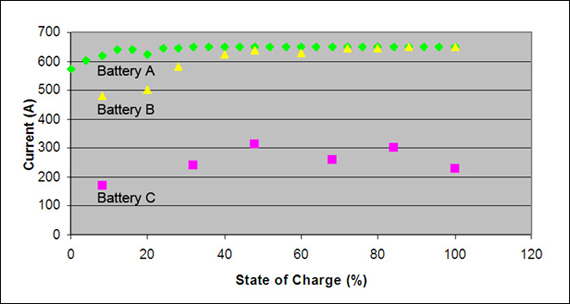
The battery condition governs accuracy. Battery A (100%) is accurate to 10% SoC; Battery B (37%) to 40% SoC. Battery C (22%) delivers unstable results.
Test condition: Batteries are discharged at 20A. CCA is measured every 10 min with Spectro™
No single instrument can evaluate all battery anomalies and rapid-testing only provides rough estimations. A micro-short in a cell, for example, can only be identified by measuring the open circuit voltage (OCV) after a rest or checking the specific gravity of the electrolyte. Rapid-testing might pass the battery as good because a charge covers up the anomaly.
All test methods encounter outliers. Reliable results are only possible if robust symptoms are present. This is not always possible with unformatted batteries and those in storage. No ideal battery test method exists but EIS has the potential of surpassing other technologies.

Looking for comments from the previous website?
Comments from the previous website are not compatible with our new commenting system but we have preserved them so our users can still reference and make use the information in them.

While manufacturing batteries what improves cca values