Propulsion by an electric powertrain is not new — Ferdinand Porsche designed a hybrid vehicle in 1898. Called the Lohner-Porsche carriage, the hybrid function served as an electrical transmission; its purpose was not to lower fuel consumption as the focus is today. With Mr. Porsche in the driver’s seat, the car broke several speed records in Austria in 1901.
Another early hybrid was the Woods Motor Vehicle built in Chicago in 1915. It had a four-cylinder internal combustion engine (ICE) in conjunction with an electric motor. Below 25km/h (15mph), the electric motor propelled the vehicle; at higher speeds the gasoline engine kicked in to take the vehicle up to 55km/h (35mph).
In early 1900, a car buyer had three choices of propulsion systems: electric, steam, and ICE, of which the ICE was the least common. The electric cars (EVs) appealed to the upper class and they were finished with fancy interiors and expensive materials. Although higher in price than the steam and gasoline-powered vehicles, the EV served the wealthy with its quiet and comfortable ride over the vibrating, smelly and maintenance-prone gasoline-powered counterpart. Best of all, the EV did not require changing gears. Back then, the knuckle-busting chore of shifting gears was the most dreaded task when driving a gasoline-powered car. Nor did the EV need manual cranking to start the engine, a task the upper class did not want to be seen doing. Since the only good roads were in town, the limited range of the EV posed no problem; most driving was local commuting.
The Detroit Electric, one of the most popular EVs then, was said to get 130km (80 miles) between battery charges. Its top speed was 32km/h (20mph), a pace considered adequate for driving. Physicians and women were the main buyers. Thomas Edison, John D. Rockefeller, Jr. and Clara Ford, the wife of Henry Ford, drove Detroit Electrics. Figure 1 shows Thomas Edison with his 1914 Detroit Electric model.

Edison felt that nickel-iron was superior to lead acid for the EV and promoted his more expensive batteries.
The battery of choice for the EV was lead acid. At a higher price point, the buyer could fit the Detroit Electric with a nickel-iron (NiFe), a battery Thomas Edison promoted for its superior cycle life and good performance at subfreezing and hot temperatures. The NiFe had a cell voltage of 1.2V, was robust and could endure overcharging and repeated full discharging but on a purely performance level, NiFe provided only a slightly better specific energy to lead acid and was expensive to manufacture. In addition, the battery had a high self-discharge of 20–40 percent per month, greater than the 5 percent with lead acid.
In 1914 a devastating fire destroyed the Edison factory, and the popularity of nickel-iron waned. Production of the EV peaked in 1912 and continued until the 1920s. Batteries already posed limitations in the electric powertrain a hundred years ago. Thomas Edison knew this and commented, “Just as soon as a man gets working on the secondary battery, it brings out his latent capacity for lying.”
Henry Ford’s mass-production and cost-cutting measures in 1912 of the Model T were not the only reason for the shift to gasoline-powered cars. The invention of the starter motor in 1912, the need to travel long distances and the discovery of Texas crude oil made the ICE more attractive and affordable to the general public.
The EV became a thing of the past until the early 1990s when the California Air Resources Board (CARB) mandated more fuel-efficient and lower-emission vehicles. It was the CARB zero-emission policy that prompted General Motors to produce the EV1. Available for lease between 1996 and 1999, the EV1 initially ran on an 18kWh lead acid battery that was later replaced with a 26kWh NiMH.
Although the NiMH had an impressive driving range of 260km (160 miles), the EV1 was not without problems. Manufacturing costs rose to three times that of a regular gasoline-powered car. In 2001, politicians changed the CARB requirements, which prompted General Motors to withdraw the EV1, to the dismay of many owners. In the 2006 documentary film Who Killed the Electric Car?, governments give a mixed message regarding cleaner transportation.
Low cost and high current capabilities make lead acid a good candidate for starter applications. It has about 720Wh, is forgiving if abused and cranks the engine even if the capacity has dropped to 30 percent. Batteries for the hybrid electric vehicle (HEV) are about twice this size, and the plug-in has about 12.5kWh; EVs go from 15kWh to 90Wh. Figure 2 compares the battery sizes.
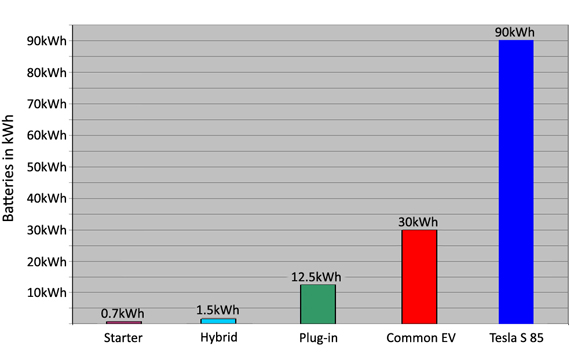
While starter and hybrid batteries are tolerant to capacity fade, a weak EV battery travels shorter distances.
Batteries and cost per kWh vary greatly according to chemistry. Table 3 estimates the price of the most common batteries in use today. At $120 per kWh, a deep-cycle-battery for golf cars and wheelchairs is most economical, followed by the starter, forklift and stationary batteries. Complex manufacturing, electronic safety circuits and battery management systems (BMS) make newer technologies more expensive than older systems, even with volume production.
| Application | Chemistry | Capacity | Cost/kWh (est.) | Battery Price |
|---|---|---|---|---|
| E-bicycle | Li-ion | 360Wh | $1,200 | $400-500 |
| Starter | Lead acid | 0.5-1kWh | $160 | $120 |
| Golf car | Lead acid | 8kWh | $120 | $720 (set) |
| Forklift | Lead acid | 18kWh | $166 | $3,000 |
| Stationary | Lead acid | Small to large | $200 | $50,000 typical |
| HEV | NiMH, Li-ion | 1-2kWh | $500 | $2,000–3,000 |
| PHEV | NiMH, Li-ion | 5–15kWh | $500 | $10,000–12,000 |
| EV | Li-ion | 20–90kWh | $350 | $10,000–30,000 |
Estimated cost/kWh is lowest with lead acid and most expensive with lithium-ion.
References
[1] Source: National Museum of American History

Looking for comments from the previous website?
Comments from the previous website are not compatible with our new commenting system but we have preserved them so our users can still reference and make use the information in them.

Why is the cost/Wh of for the Ebike battery 10 times what it is for EVs? Is it not the same technology?