All batteries are affected by self-discharge. Self-discharge is not a manufacturing defect but a battery characteristic; although poor fabrication practices and improper handling can increase the problem. Self-discharge is permanent and cannot be reversed. Figure 1 illustrates self-discharge in the form of leaking fluid.
Self-discharge increases with age, cycling and elevated temperature. Discard a battery if the self-discharge reaches 30 percent in 24 hours.
The amount of electrical self-discharge varies with battery type and chemistry. Primary cells such as lithium-metal and alkaline retain the stored energy best, and can be kept in storage for several years. Among rechargeable batteries, lead acid has one of the lowest self-discharge rates and loses only about 5 percent per month. With usage and age, however, the flooded lead acid builds up sludge in the sediment trap, which causes a soft short when this semi-conductive substance reaches the plates(See BU-804a: Corrosion, shedding and Internal Short)
The energy loss is asymptotical, meaning that the self-discharge is highest right after charge and then tapers off. Nickel-based batteries lose 10–15 percent of their capacity in the first 24 hours after charge, then 10–15 percent per month. Figure 2 shows the typical loss of a nickel-based battery while in storage.
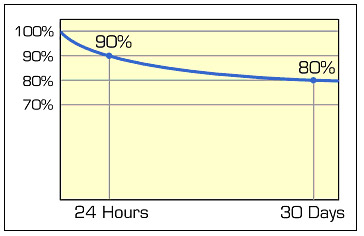
The self-discharge is highest right after charge and tapers off. The graph shows self-discharge of a nickel-based battery. Lead- and lithium-based systems have a lower self-discharge.
NiMH and NiCd belong to rechargeable batteries that have the highest self-discharge; they need recharging before use when placed on a shelf for a few weeks. High-performance NiCd has a higher self-discharge than the standard versions. Furthermore, the self-discharge increases with use and age, of which crystalline formation (memory) is a contributing factor. Regular full discharge cycles keeps memory under control(See BU-807: How to restore Nickel-based Batteries)
Li-ion self-discharges about 5 percent in the first 24 hours and then loses 1–2 percent per month; the protection circuit adds another 3 percent per month. A faulty separator can lead to elevated self-discharge that could develop into a current path, generating heat and, in an extreme case, initiate a thermal breakdown. In terms of self-discharge, lead acid is similar to Li-ion. Table 3 summarizes the expected self-discharge of different battery systems.
| Battery System | Estimated Self-Discharge |
|---|---|
| Primary lithium-metal | 10% in 5 years |
| Alkaline | 2–3% per year (7-10 years shelf life) |
| Lead-acid | 10–15% in 24h, then 10-15% per month |
| Nickel-based | Li-ion, NiCd, NiMH |
| Lithium-ion | 5% in 24h, then 1–2% per month (plus 3% for safety circuit) |
Primary batteries have considerably less self-discharge than secondary (rechargeable) batteries.
The self-discharge of all battery chemistries increases at higher temperature, and the rate typically doubles with every 10°C (18°F). A noticeable energy loss occurs if a battery is left in a hot vehicle. High cycle count and aging also increase self-discharge of all systems. Nickel-metal-hydride is good for 300–400 cycles, whereas the standard nickel-cadmium lasts for over 1,000 cycles before elevated self-discharge starts interfering with performance. The self-discharge on an older nickel-based battery can get so high that the pack goes flat from leakage rather than normal use(See BU-208: Cycling Performance demonstrating the relationship of capacity, internal resistance and self-discharge)
Under normal circumstances the self-discharge of Li-ion is reasonably steady throughout its service life; however, full state-of-charge and elevated temperature cause an increase. These same factors also affect longevity. Furthermore, a fully charged Li-ion is more prone to failure than one that is partially charged. Table 4 shows the self-discharge per month of Li-ion at various temperatures and state-of-charge. The high self-discharge at full state-of-charge and high temperatures comes as a surprise(See BU-808: How to Prolong Lithium-based Batteries)
| Type | 0°C (32°F) | 25°C (77°F) | 60°C (140°F) |
|---|---|---|---|
| Full Charge | 6% | 20% | 35% |
| 40–60% Charge | 2% | 4% | 15% |
Self-discharge increases with rising temperature and higher SoC.
Lithium-ion should not be discharged below 2.50V/cell. The protection circuit turns off and most chargers will not charge the battery in that state. A “boost” program applying a gentle charge current to wake up the protection circuit often restores the battery to full capacity(See BU-803a: How to Awaken Sleeping Li-ion)
There are reasons why Li-ion is put to sleep when discharging below 2.50V/cell. Copper dendrites grow if the cell is allowed to dwell in a low-voltage state for longer than a week. This results in elevated self-discharge, which could compromise safety.
Self-discharge mechanisms must also be observed in manufacturing. They vary from corrosion to impurities in the electrodes that reflect in self-discharge variations not only from batch to batch but also form cell to cell. A quality manufacturer checks the self-discharge of each cell and rejects those that fall outside tolerances.
Regular charge and discharge causes an unwanted deposit of lithium metal on the anode (negative electrode) of Li-ion, resulting in capacity loss through a depletion of the lithium inventory and the possibility of creating an internal short circuit. An internal short is often preceded with elevated self-discharge, a field that needs further research to learn what levels of self-discharge would pose a hazard that can lead to a thermal runaway. Unwanted lithium deposition also increases the internal resistance that reduces loading capability.
Figure 5 compares the self-discharge of a new Li-ion cell with a cell that underwent forced deep discharges and one that was fully discharged, shorted for 14 days and then recharged. The cell that was exposed to deep discharges beyond 2.50V/cell shows a slightly higher self-discharge than a new cell. The largest self-discharge is visible with the cell that was stored at zero volts.
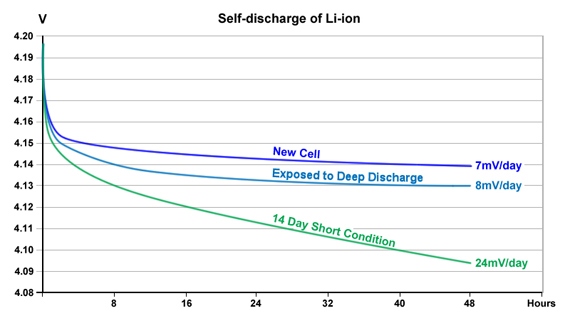
Cells that had been stressed with deep discharges and kept at 0V show a higher self-discharge than a new cell.
Figure 6 illustrates the self-discharge of a lead acid battery at different ambient temperatures At a room temperature of 20°C (68°F), the self-discharge is roughly 3% per month and the battery can theoretically be stored of 12 months without recharge. With a warm temperature of 30°C (86°F), the self-discharge increases and a recharge will be needed after 6 months. Letting the battery drop below 60 percent SoC for some time causes sulfation(See also BU-702: How to Store Batteries)
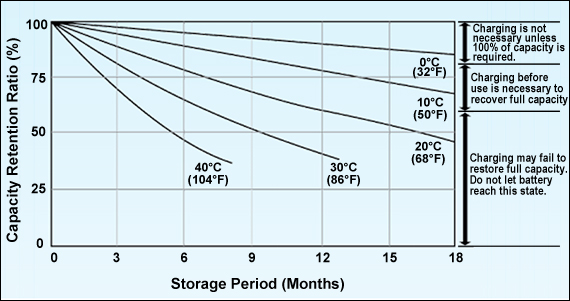
Lead acid should never drop below 60% SoC. Charge more often when warm.
Reference
[1] Courtesy of Cadex
[2] Source: TU München
[3] Source: Power-Sonic

Comments
I read once that submarine lead acid batteries were found to self discharge due to the difference in temperature between their base which was in contact with the cold hull and the warmer room temperature, causing a circulating flow in the electrolyte. So the engineers insulated them from the hull. Is this true and would it apply to vehicle batteries stored on a cold floor?
Looking for comments from the previous website?
Comments from the previous website are not compatible with our new commenting system but we have preserved them so our users can still reference and make use the information in them.

Don Whyte, this is fascinating. And.. I wish I had an educated answer. I sure am curious to learn if you found out anything as surprisingly it appears there are no comments or responses yet.